NEW YORK and LONDON, May 19, 2022 (GLOBE NEWSWIRE) -- Akari Therapeutics, Plc (Nasdaq: AKTX), a late-stage biotechnology company focused on developing advanced …
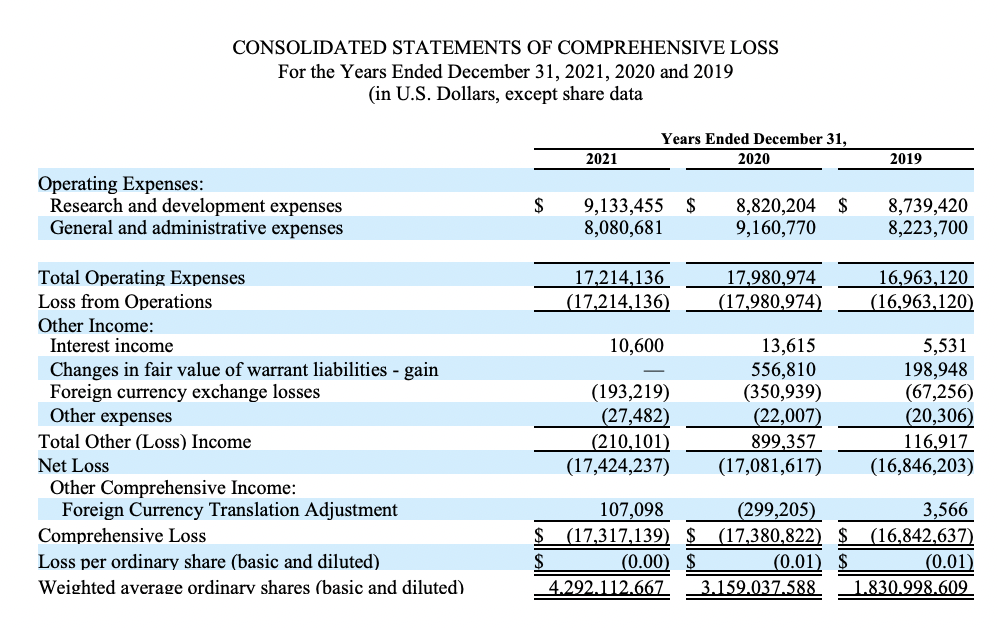
Akari Therapeutics Reports Full Year 2021 Financial Results and Highlights Clinical Progress
Clinical trial sites open and enrolling patients in FDA and EMA registration-directed Phase III Part A study of nomacopan in pediatric hematopoietic stem cell …
Akari Therapeutics to Present Results from Compassionate Use of Nomacopan for COVID-19 (CORONET) and Identification of Clinical Deterioration Risk in COVID-19 (CASCADE) at the American Thoracic Society (ATS) 2022 Annual Meeting
NEW YORK and LONDON, May 11, 2022 (GLOBE NEWSWIRE) -- Akari Therapeutics, Plc (Nasdaq: AKTX), a late-stage biotechnology company …
Akari Therapeutics Announces Publication of Phase II Data of Investigational Nomacopan for the Treatment of Bullous Pemphigoid (BP) in JAMA Dermatology
• Patients received subcutaneous nomacopan (30 mg once daily) with lesional mometasone from Day 1 to 21 of treatment and nomacopan only …
Akari Presents Results from Two Preclinical Development Programs of Long-Acting PAS-Nomacopan in Geographic Atropy (GA) Dry Age-Related Macular Degeneration and Nomacopan in Experimental Immune-Mediated Conjunctival Disease (EIC)
• Preclinical data show investigational intravitreal long-acting PASylated-nomacopan (PAS-nomacopan) effective in inhibiting choroidal neovascularization (CNV), …